Vitamin B9
VitB9
Folate, Tetrahydrofolate(THF), 5,10-methylene THF, 10-formyl THF, 5-formimino THF, 5,10-methylenyl THF, 5-methyl THF
Daily Requirement:
Modified DV:
RDA ?:
Adequate Intake ?:
400
true
DFE/d
DFE/d
Min Deficiency:
Max Toxicity:
Tolerable UL
Animal:Plant Conv:
1000
DFE/d
DFE/d
DFE/d
Date Discovered:
1941
Short Description:
One-carbon transfer reactions for nutrient metabolism and energy production; gene expression; purien and pyrimidine synthesis for DNA and RNA; hematopoiesis. Major Food Sources: Green vegetables = spinach, asparagus, and greens; legumes; fortified grain products. RDA: 400 mcg
Interpretation:
Main sources:
Animal Products: Liver, dairy products, egg yolks
Plant Products: Oranges, Dark leafy vegetables, peanuts & beans
Dietary methyl donors and DNA methylation
• What nutrients are considered methyl donors
Single carbon metabolation pathway, folate, zn, b6, b12, methionine, choline
• Where does DNA methylation normally occur
CpG islands, promoters of genes. folate deficiency has - dutch hunger study - starvation causes problems later in life, first trimester - igf2 dna methylation lack. folate deficiency uracil misincorporation. If folate deficiency, uracil can't turn into thymine. Causes strand breaks, two uracil residues = double strand breaks - bad.
• What is the significance of the agouti mouse experiments
Dietary exposure of your grandma can effect your genotype. Your egg was exposed to that that diet.
• Why are dietary methyl donors important in cancer, what are the mechanisms
If low methyl donors, aberrant methylation, chance for uracil -> cancer
History & Discovery:
Digestion:
Jejunum is the main site of digestion and absorption.
Food folates with many glutamine (3 or 7) need to be broken down by proton-coupled folate transporter PCFT in the membrane of the enterocyte. Then the food folate with one Glu is brought into the enterocyte as either THF or 5,10-methylene-THF, which goes to 5-methyl-THF for transport to the Plasma through multidrug resistant-associated protein (MRP).
Stored in small quantities in liver, kidneys, and spleen for 3-4 months.
Uptake into tissues requires proton-coupled folate transporter (PCFT) or organic anion transporting polypeptides (OATP) B1 and B3.
5-methyl-THF is the main form in blood plasma.
Folate is found as free (33%) or bound to albumin and alpha2-macroglobin (66%)
Absorption and Storage:
AI of folate = 320 mcg/day.
Folic acid from supplements/fortified foods is more bioavailable. This is taken into account for Dietary Folate Equivalents (DFE).
1 DFE = 1 mcg of food folate = 0.5 mcg folic acid from supplement on empty stomach
1 DFE = 1 mcg of food folate = 0.6 mcg folic acid from fortified food (1.7 factor)
Example:
Half cup of avocodo = 60 mcg of folate
Slice of bread = 50 mcg of folic acid * 1.7 = 85 mcg
Folate is degraded by heat, oxidation and UV light.
Overall absorption is 10-90% or 50% on average due to food matrix and inhibitors.
Folate bioavailability of beef liver, lima beans, peas, spinach, mushrooms, collards, orange juice and wheat germ was estimated with a protocol of folate depletion-repletion using growth and liver, serum and erythrocyte folate of weanling male rats. Diets with 125, 250 and 375 micrograms folic acid/kg were standards. Individual foods were incorporated into a folate-free amino acid-based diet alone (250 micrograms folate/kg diet from food) or mixed with folic acid (125 micrograms folate from food + 125 micrograms folic acid) to evaluate folate bioavailability and effects of food matrix. Beef liver and orange juice folates were as available as folic acid, whereas those of wheat germ were less bioavailable. Folates of peas and spinach were also less available than folic acid using liver and serum folate concentrations and total liver folate as response criteria, but they were not lower when based on growth and erythrocyte folate concentrations. Lima bean, mushroom and collard folates were as available as folic acid using four of five response criteria. Folate bioavailability of all foods generally exceeded 70%. All response criteria gave approximately equivalent results, indicating that growth and tissue folate levels are appropriate criteria. No food matrix effects were observed for any food except lima beans. Foods rich in polyglutamyl folates were less bioavailable than those of foods rich in short-chain folates. https://pubmed.ncbi.nlm.nih.gov/2007897/
Folic acid, 5-formyl THF and 5-methyl THF absorbed intact.
Folate in food needs to be converted to tetrahydrofolate (monoglutamate form) by folate hydrolase, which requires Zn.
Jejunum is the main site of digestion and absorption.
Important Pathways:
Folate Appearance
Folate Forms
Folate = tetrahydrofolate (food form) + folic acid (oxidized version, found in supplement form)
Humans can synthesize each individual part but we cannot link pteridine to PABA
One glutamic acid on figure, but typically attached to 3 or 7 glutamic acids in the body and in food which is called a 'glu tail'. 75% of folate exists in this form.
Function of Folate:
One carbon transfer or methyl donor. "Dumping" a methyl on B12 (CH3), which can then be dumped onto homocysteine to be turned into S-adenosyl methionine = central methyl donor which is important for anabolic reactions.
DNA Synthesis
5,10-methyleneTHF reacts with dUMP to create dTMP which can be used for DNA synthesis. Or it can become 5-methylTHF to react with homocysteine to become methionine which can enter the SAM cycle to lead to DNA methylation.
Reactions between Forms of Folate
SAM Cycle
Homocysteine -> Cystathione -> Leads to succinyl CoA, glucose, glycine, Heme
Deficiency Diseases, Detection, Cures:
Red blood cells normally divide, yet this doesn't happen with folate or B12 deficiency.
Deficiency disease:
Common in early 1900's in young. female workers in former British colonies.
Tropical megaloblastic anemia - hyperpigmentation of fingers before treatment of anaemia (A)
Reduction 3 months after treatment (B)
Peripheral blood film shows hypersegmented neutrophils (green arrowheads), macrocytes (blue arrowheads), and a teardrop shaped red blood cell (red arrowhead). (C)
Erythropoiesis: maturation of red blood cells
Hematopoietic stem cell -> Proerythroblast -> Megaloblastic red blood cell due to folate deficiency -> Erythroblasts - two cytosols but no nuclei division (requires carbon donor folate) -> Large immature red blood cells -> Megaloblastic anemia - fewer red blood cells to take up iron -> heme iron deficiency
Recommendation: Take 400 mcg DFE/day 1-3 months before pregnancy and first month to help with embryonic neural tube closure to prevent anencephaly.
Symptoms of Deficiency:
cardiomegaly, tachycardia, shortness of breath
megaloblastic anemia
Genetic Diseases:
Dietary methyl donors and DNA methylation
• What nutrients are considered methyl donors?
• Where does DNA methylation normally occur?
• What is the significance of the agouti mouse experiments?
• Why are dietary methyl donors important in cancer, what are the mechanisms?
Higher eukaryotes use epigenetic modifications to reversibly suppress transcription of genes and repeat elements, often by stable silencing
Epigenetic marks are retained through mitosis, allowing maintenance of characteristic cell types
By their nature, epigenetic modifications are susceptible to environmental influence: the interposition of epigenetic modifications between genes and the environment provides a way in which the environment can exert heritable influences on phenotype
Methylation of DNA
Promoter regions of many genes are rich in CpG dinucleotides
Carbon 5 of cytosine in CpG dinucleotides is a prime target for methylation by DNA methyltransferases (DNMT)
Depending on cell type or tissue, 3–4% of all cytosines in vertebrate DNA and 70% of cytosines in CpG dinucleotides are 5-methylated
Methylation of cytosines is a heritable modification of DNA. However, the following mechanisms are available to remove methylation marks in vivo:
enzyme-mediated removal of methyl groups
passive removal of methyl groups if methylation is not maintained at the time of replication
DNMT utilize S-adenosylmethionine (SAM) as a methyl donor
Folate plays a unique role in the generation of SAM, methylentetrahydrofolate reductase catalyzes the conversion of 5,10-methylenetetrahydrofolate to 5- methyltetrahydrofolate
The latter donates a methyl group to convert homocysteine to methionine
Methylenetetrahydrofolate reductase deficiency results in hypomethylation of DNA, consistent with a role for folate in DNA methylation
Nutrients other than folate also play roles in one-carbon metabolism: choline and methionine are the major dietary sources of methyl groups
whereas vitamins B-6 and B-12, riboflavin and zinc are coenzymes and cofactors, respectively, for various steps in one-carbon metabolism
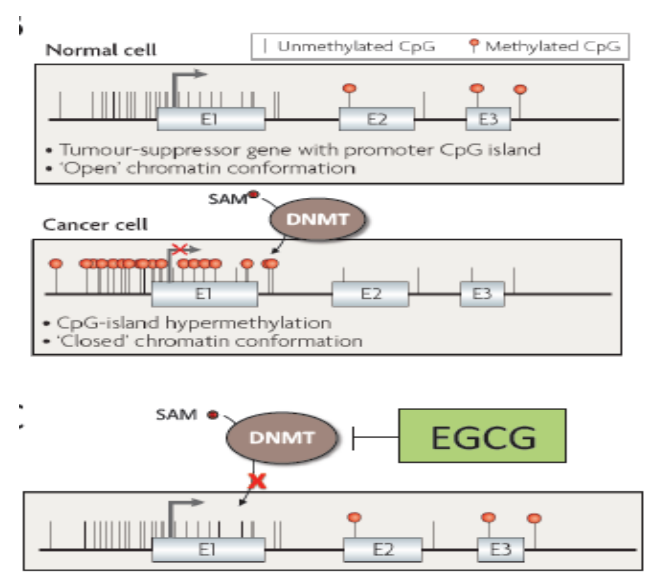
Methylation of CpG islands is associated with transcriptional repression and provides a mean to control gene expression
DNA methylation plays a role in allele-specific gene expression (genomic imprinting), heritable transcriptional silencing of parasitic sequence elements and X-chromosome inactivation
Impaired DNA methylation is associated with perinatal death, decreased fertility, abnormal fetal development and tumorigenesis
Histone and DNA Methylation
Effect of Methyl Donors on the Epigenetic Process
Several observations point to the ability of several environmental factors, including diet, to be key regulators of epigenetic processes, and evidence exists for diet effects on both DNA methylation and histone posttranslational modulation
Diet can influence the degree of methylation by influencing the availability of methyl donors, including folate, choline, and methionine
Several dietary factors ranging from alcohol to zinc have been reported to influence the supply of methyl groups for the formation of SAM and thereby affect the methylation of CpGs
Dietary methyl deficiency (of folate, choline, and methionine) in a rat model is known to alter hepatic DNA methylation patterns and induce hepatocarcinogenesis in the absence of a carcinogen
Dietary components such as EGCG found in green tea can influence gene methylation
Gene Imprinting and Diet
Genomic imprinting is an epigenetic modification that inactivates one allele of a gene in a parent-of-origin-dependent manner. It occurs primarily by allelic-specific methylation of cytosines in CpG dinucleotides during gametogenesis
Although relatively few in number, imprinted genes are thought to be vital to human development, and their dysregulation appears to affect the risk of developing some diseases
One example by which early dietary exposures may affect genomic imprinting involves the imprinted gene insulin-like growth factor 2 (IGF2)
The maternally inherited allele of the imprinted gene encoding IGF-2 is normally epigenetically silenced, resulting in expression almost exclusively from the paternal allele
Interestingly, loss of imprinting at the IGF2 locus has been shown to induce biallelic expression of this growth factor in 10% of normal human adults and is implicated in several types of cancer
For the most part, the cause of the IGF2 loss of imprinting is unknown
Mice weaned on either a synthetic control or a synthetic methyl donorcofactor deficient diet compared with a standard diet displayed hypomethylation of IGF2 and consequent dysregulation of IGF2 allelic expression
The loss of imprinting caused by the deficient diet persisted during a subsequent 100-d recuperation period after the mice were switched to the control diet
Thus, early nutritional influences may stimulate changes in cytosine methylation to which imprinted genes, such as IGF2, may be susceptible
These findings in view of the relation between IGF-2, insulin, and metabolic syndrome suggest that early nutrition may influence susceptibility to adult obesity, diabetes, cardiovascular disease, and cancer
Dutch Hunger Winter Study - Heijmans B. T. et.al. PNAS 2008;105:17046-17049
Difference in IGF2 DMR methylation between individuals prenatally exposed to famine and their same-sex sibling. Periconceptional exposure: Difference in methylation according to the mother's last menstrual period (a common estimate of conception) before conception of the famine-exposed individual.
Methyl Donors, Nutrition and Cancer
In comparison to normal differentiated cells, tumor cells are characterized by a global hypomethylated state despite overexpression of DNA methyltransferase, although they have distinct regions of hypermethylation
Even though this process and the influence of inadequate dietary supply of methyl donors and/or genetic predisposition to aberrant methylation have been extensively studied, the precise mechanisms underlying the methylation aberrations in cancer are poorly understood
Neither is it known whether global hypomethylation and regional hypermethylation are causally linked or are completely independent events occurring in the same cellular context of malignant transformation
The prevailing hypothesis is that hypermethylation of promoter-associated CpG islands of tumor suppressor genes leads to inactivation of these genes and development or progression of cancer
Promoter hypermethylation has been observed in genes that play a role in cell cycle regulation, apoptosis, DNA repair and replication, angiogenesis, cell differentiation, and other processes that, when uncontrolled, can promote carcinogenesis
Silencing these genes can be a second hit in the two-step carcinogenesis process
Global DNA hypomethylation has been measured in many cancer cells and primary tumors from humans and animal models
Chromosomal instability and DNA double-strand breaks have been demonstrated in laboratory rats fed hypomethylated diets, in people with methyl-donor deficient diets and in cancer cells
Hypomethylation could lead to activation of oncogenes. In vivo studies of rats fed methyl-donor deficient diets showed that overexpression of oncogenes such as cmyc, c-fos, and c-ha-ras
When diets were methyl-donor replete, the DNA methylation reverted to normal, but not for all cytosines that were examined. This suggests that DNA methylation defects can be irreversible after prolonged exposure to diets low in methyl donors
Increased sensitivity of rats to known carcinogens has also been observed when diets are methyl-donor deficient
In humans a good correlation between reduced tissue and plasma folate levels and hypomethylation of both normal and tumor tissues was demonstrated in patients with cervical cancer
A large prospective study in humans also showed that methyl donor deficiency correlates with an increased risk for tumors of the liver and colon and demonstrated an inverse relationship between DNA methylation of colon adenomas and dietary folate
Such studies further suggest that this risk may be aggravated by other risk factors, such as alcohol intake and smoking. Polymorphisms in the folate metabolic enzymes, e.g., the MTHFR 677C>T also influence cancer risk
Because of the variability in methylation within one tumor or between tumor types, the potential benefit of methylation diets in cancer treatment or prevention, versus treatment with demethylating agents, may be highly variable
Folate and DNA Stability
Dietary Folate and DNA Stability
Conversion of dUMP to dTMP is dependent on the presence of N5 N10 Methylene THF
Folate deficient cultured cells
6X increase in dUTP/dTTP ratio after 11 days folate deficiency
3 days folate repletion corrected imbalance (Melnyk et al., 1999)
Catastrophic repair cycle- (Dianov et al., 1991)
Uracil removed by uracil-DNA glycosylase, strand break
Uracil residues within 14 bp on opposing strands, double strand break
DNA from folate deficient individuals
9X more uracil residues than folate sufficient individuals
50X more likely to have double strand breaks (Blount et al., 1997)
Dietary Folate and Cancer
Nurses health study (N = 88,757): 15 yrs of 400 µg folate/day resulted in 75% reduction in colon cancer incidence (Giovanucci et al., 1998)
At least 5 major case/control and 5 prospective studies have found lowered colorectal cancer risk with increased dietary folate (Giovannuci et al., 2002)
Epidemiological evidence demonstrates fruit and vegetable consumption is inversely related to colon cancer is hypothesized to be related to folate intake (Ames, 1998)
Folate intake is initially protective in animal colon cancer models (Song et al., 2000; Trasler et al., 2003)
The Avy allele and the Agouti mouse model
The Avy allele carries an insertion of an IAP retrotransposon in an antisense direction in agouti pseudoexon
The Avy transcript originates from a cryptic promoter in the 5'LTR of the IAP and is spliced to agouti coding exons 2, 3 and 4, which encode ASP
When the IAP is silent, agouti is transcribed from hair-cycle-specific promoters in exons 1B and 1C
Avy phenotypes are scored from 1 to 5 based on coat color. Fully yellow mice are scored as 1, and fully agouti mice are scored as 5. Phenotypes of mosaic mice range from mostly yellow (2) to mottled yellow/agouti (3) to mostly agouti (4).
Avy/a mice vary in coat color phenotype from clear yellow (CY), to slightly mottled yellow (SMY), mottled yellow (MY), heavily mottled yellow (HMY), and pseudoagouti (PA)
The top panel shows percentage methylation of 7 CpG sites in the Avy region in Avy/a mice according to the coat color classifications displayed in the bottom panel
Methylation of the Avy locus is responsive to nutrition and tightly correlated with coat color phenotype, rendering the Avy/a mouse useful as an epigenetic biosensor.
Your Grandma's Diet & Epigenetics
(F1) pseudoagouti Avy/a females that had been exposed to methyl donors in utero were mated to a/a males without any further methyl donor supplementation
This strategy takes advantage of the tendency for the Avy epigenotype to be partially stable in the female germ line
The phenotypes of the second generation (F2) offspring were compared with phenotypes of pups born to pseudoagouti females with no history of exposure to methyl donor supplementation
Phenotypes of these F2 mice were significantly shifted toward the pseudoagouti
Thus a pseudoagouti dam who was exposed to methyl donor supplementation only when she was in utero gives rise to phenotypically different offspring than does an otherwise (genetically and phenotypically) identical female who had no exposure to methyl donor supplementation
This grandparental effect is directly attributable to the epigenetic state of the Avy allele.